

Again, if no precipitate is formed in the cold, it may be necessary to warm the mixture very gently. Potassium iodide solution is added to a small amount of an alcohol, followed by sodium chlorate(I) solution. Sodium chlorate(I) is also known as sodium hypochlorite. What does this mean for Arizona Precipitation records over the last few decades in Arizona show dramatic swings from very wet one year to very dry the next. Using potassium iodide and sodium chlorate(I) solutions.It is used as an antiseptic on the sort of sticky plasters you put on minor cuts, for example. Iodoform (CHI3) is an alternative candidate radiopacifier whose impact on the setting, bioactivity, antimicrobial properties and cytotoxicity of white Portland.
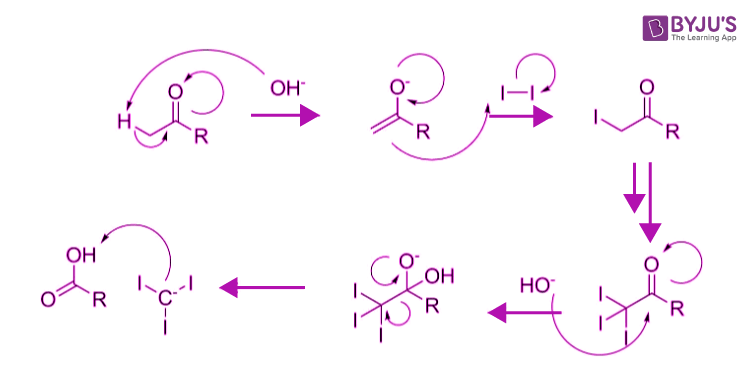
The iodoform reaction been used as a chemical test for the presence of a methylketonemoiety. The halo form reaction only works if you have a methyl ketone, methyl secondary alcohol, ethanol or ethanal present. a yellow solid which may precipitatefrom the reaction mixture. After standing for 15 minutes, a pale yellow precipitate of iodoform (mp 119-121oC) is a positive test for a methyl ketone.

It is used widely for identifying aldehydes or ketones. If a precipitate is present, it means a positive result. If sodium hydroxide and Iodine are mixed with a secondary alcohol compound or a methyl ketone in the alpha position, it produces a yellow precipitate of triiodomethane or Iodoform. Potassium iodide and sodium hypochlorite solution are added to the organic compound in the presence of sodium hydroxide solution. A positive result is the appearance of a very pale yellow precipitate of triiodomethane (previously known as iodoform): CHI 3, which apart from its color, can also be recognized by its faintly "medical" smell. It depends on what you mean by mild white turbidity. The iodoform test is a test to determine if an organic compound is ethanol or contains a secondary alcohol (CH 3 CH (OH)R). What is a halogen, you may ask Any of the six nonmetallic elements that comprise Group 17 of the periodic table are halogens. If nothing happens in the cold, it may be necessary to warm the mixture very gently. CBSE Notes What is Iodoform Iodoform is a yellow, crystalline solid belonging to the family of organic halogen compounds. Iodine solution is added to a small amount of an alcohol, followed by just enough sodium hydroxide solution to remove the color of the iodine. This is chemically the more obvious method. 2019 The iodoform reaction is greatly retarded by steric hindrance. Some alcohols, if not purified, may contain aldehyde or ketone impurities. Some allylic alcohols are oxidized by the reagent to aldehydes and give a positive test. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor and analogous to chloroform, sweetish taste. Complications Some ketones give oils which will not solidify. definition Iodoform Iodoform is the organoiodine compound with the formula CHI. There are two apparently quite different mixtures of reagents that can be used to do this reaction, but are chemically equivalent. Iodoform Test: Learn Definition, Mechanism and Test for Alcohols Nettet20. Formation of a precipitate is a positive test. The triiodomethane (iodoform) reaction can be used to identify the presence of a CH 3CH(OH) group in alcohols. Thus, at any intermediate an acetate anion could generate inhibiting any further reactions.\) It is also to be noted that an ester can always be hydrolysed to the corresponding acid under basic aquaeous conditions. Formation of a yellow precipitate on heating a compound with an alkaline solution of iodine is known as iodoform reaction. N/10 thiosulphate or iodine (mean of duplicate. The attack of a nucleophilic (hydroxide) species to the resulting anion is strongly disfavoured. the filtrate from the iodoform, the precipitated barium sulphate and. The resulting acid is merely deprotonated. It also tests positive for a few specific. The $\mathrm$ value of both’s conjugate acids. The reaction of iodine, a base and a methyl ketone gives a yellow precipitate along with an antiseptic smell. This can fail for carboxylic acids or esters in one of two ways: (The final proton transfer need not occur between the two partners, any other hydroxide could abstract the acid’s proton and any water molecule can protonate iodoform.) The iodoform reaction proceeds by the mechanism shown below.


 0 kommentar(er)
0 kommentar(er)
